Author: Shigeo Ohta
Associate editor: Ikuko Kimura
Abstract
Molecular hydrogen (H2) has been accepted to be an inert and nonfunctional molecule in our body. We have turned this concept by demonstrating that H2 reacts with strong oxidants such as hydroxyl radical in cells, and proposed its potential for preventive and therapeutic applications. H2 has a number of advantages exhibiting extensive effects: H2 rapidly diffuses into tissues and cells, and it is mild enough neither to disturb metabolic redox reactions nor to affect signaling reactive oxygen species; therefore, there should be no or little adverse effects of H2. There are several methods to ingest or consume H2; inhaling H2 gas, drinking H2-dissolved water (H2-water), injecting H2-dissolved saline (H2-saline), taking an H2 bath, or dropping H2-saline into the eyes. The numerous publications on its biological and medical benefits revealed that H2 reduces oxidative stress not only by direct reactions with strong oxidants, but also indirectly by regulating various gene expressions. Moreover, by regulating the gene expressions, H2 functions as an anti-inflammatory and anti-apoptotic, and stimulates energy metabolism. In addition to growing evidence obtained by model animal experiments, extensive clinical examinations were performed or are under investigation. Since most drugs specifically act to their targets, H2 seems to differ from conventional pharmaceutical drugs. Owing to its great efficacy and lack of adverse effects, H2 has promising potential for clinical use against many diseases.
[…]
Discovery of the biological effects of molecular hydrogen
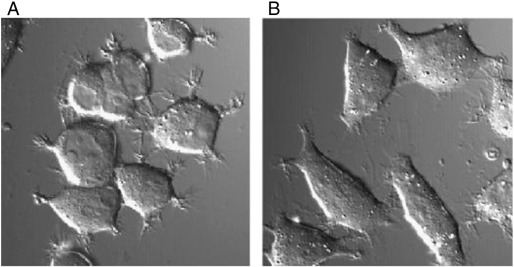
During the process of searching for the ideal antioxidant, we infused H2 into culture medium without changing pH, O2 and CO2 concentrations or other conditions. When cultured PC12 cells were exposed to oxidative stress by treatment with antimycin A, an inhibitor of the mitochondrial electron translocation chain, the cells apparently shrank and extended short fibers in response to oxidative stress (Fig. 2A). In contrast, when the cells were treated with the inhibitor in the presence of H2, the cells did not change their shape (Fig. 2B). In H2-degassed medium from H2-medium, the cells again responded to oxidative stress. This finding indicates that H2 affected no components in the original medium, but directly acted to the cells. By this finding of the first experiment, we foresaw that H2 has great potential for actual clinical use.
After this experiment, we tried to identify the target of H2 in cultured cells. H2 dissolved in culture medium did not change the cellular levels of O2 − and H2O2, as judged by the fluorescent signals of MitoSOX and dichlorofluorescein-diacetate (DCF-DA), respectively. Additionally, H2 did not decrease the cellular level of
− and H2O2, as judged by the fluorescent signals of MitoSOX and dichlorofluorescein-diacetate (DCF-DA), respectively. Additionally, H2 did not decrease the cellular level of  NO. In contrast, H2 treatment significantly decreased levels of
NO. In contrast, H2 treatment significantly decreased levels of  OH, as judged by the decrease in the fluorescent signal of hydroxyphenyl fluorescein (HPF) (Setsukinai et al., 2003). Moreover, the decrease in the cellular
OH, as judged by the decrease in the fluorescent signal of hydroxyphenyl fluorescein (HPF) (Setsukinai et al., 2003). Moreover, the decrease in the cellular  OH level by H2 was confirmed by spin trapping technology (Halliwell & Gutteridge, 1992).
OH level by H2 was confirmed by spin trapping technology (Halliwell & Gutteridge, 1992).
 − and H2O2, as judged by the fluorescent signals of MitoSOX and dichlorofluorescein-diacetate (DCF-DA), respectively. Additionally, H2 did not decrease the cellular level of
− and H2O2, as judged by the fluorescent signals of MitoSOX and dichlorofluorescein-diacetate (DCF-DA), respectively. Additionally, H2 did not decrease the cellular level of  NO. In contrast, H2 treatment significantly decreased levels of
NO. In contrast, H2 treatment significantly decreased levels of  OH, as judged by the decrease in the fluorescent signal of hydroxyphenyl fluorescein (HPF) (Setsukinai et al., 2003). Moreover, the decrease in the cellular
OH, as judged by the decrease in the fluorescent signal of hydroxyphenyl fluorescein (HPF) (Setsukinai et al., 2003). Moreover, the decrease in the cellular  OH level by H2 was confirmed by spin trapping technology (Halliwell & Gutteridge, 1992).
OH level by H2 was confirmed by spin trapping technology (Halliwell & Gutteridge, 1992).The selective reduction of ROS can be explained by the marked oxidative strength of  OH, as shown in Fig. 3, which was profiled according to published data (Setsukinai et al., 2003). This means that
OH, as shown in Fig. 3, which was profiled according to published data (Setsukinai et al., 2003). This means that  OH is strong enough to react with even inert H2, but that O2
OH is strong enough to react with even inert H2, but that O2 −, H2O2, and
−, H2O2, and  NO are insufficient to react with H2 according to their activities. In other words, H2 is mild enough neither to disturb metabolic redox reactions nor to affect ROS that function in cellular signaling.
NO are insufficient to react with H2 according to their activities. In other words, H2 is mild enough neither to disturb metabolic redox reactions nor to affect ROS that function in cellular signaling.
 OH, as shown in Fig. 3, which was profiled according to published data (Setsukinai et al., 2003). This means that
OH, as shown in Fig. 3, which was profiled according to published data (Setsukinai et al., 2003). This means that  OH is strong enough to react with even inert H2, but that O2
OH is strong enough to react with even inert H2, but that O2 −, H2O2, and
−, H2O2, and  NO are insufficient to react with H2 according to their activities. In other words, H2 is mild enough neither to disturb metabolic redox reactions nor to affect ROS that function in cellular signaling.
NO are insufficient to react with H2 according to their activities. In other words, H2 is mild enough neither to disturb metabolic redox reactions nor to affect ROS that function in cellular signaling.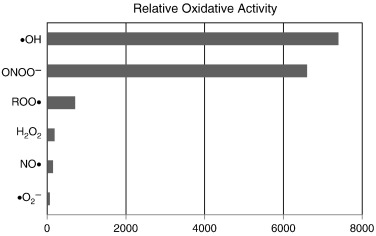
Effects of molecular hydrogen and clinical examinations
1. Safety of molecular hydrogen for humans
H2 has advantages from the aspect of toxicity: H2 has no cytotoxicity even at high concentration (Abraini et al., 1994, Lillo et al., 1997, Fontanari et al., 2000, Lillo and Parker, 2000). Safety standards have been established for high concentrations of H2 gas for inhalation since high-pressure H2 gas is used in deep-diving gas mixes to prevent decompression sickness and arterial gas thrombi (Fontanari et al., 2000). Importantly for clinical use, the safety of H2 for humans is demonstrated by its application of an extremely high concentration of H2 gas in Hydreliox, an exotic, breathing gas mixture of 49% H2, 50% helium and 1% O2, which is used to prevent decompression sickness and nitrogen narcosis during very deep technical diving (Abraini et al., 1994, Lillo et al., 1997, Fontanari et al., 2000, Lillo and Parker, 2000). Considering that H2 is an inert gas and nonfunctional in our body, its lack of toxic effects is easily understandable. As mentioned above, inhalation of 1–4% H2 gas exhibits great efficacy (Ohsawa et al., 2007, Hayashida et al., 2008).
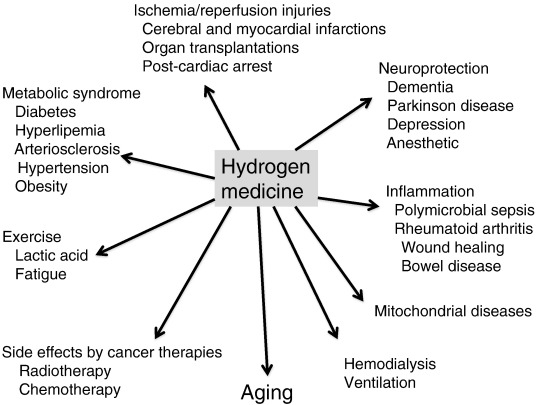
Many reports have supported that H2 exhibited great efficacy in extensive disease models regardless of the ingestion methods of H2 as summarized in Fig. 4. This review article focuses mainly on clinical examinations.
2. Protective effects against reperfusion injury
Ischemia/reperfusion induces serious oxidative stress, and its injuries should be considered in many clinical treatments. Inhalation of H2 gas improved ischemia/reperfusion injuries in cerebral (Ohsawa et al., 2007) and myocardial infarction (Hayashida et al., 2008, Yoshida et al., 2012). Hydrogen-saline protected renal ischemia-reperfusion injury (Wang et al., 2011). All clinical manifestations related to post-cardiac arrest (CA) syndrome are attributed to ischemia/reperfusion injury in various organs, including the brain and heart. H2 gas inhalation yielded great improvement in survival and the neurological deficit score in post-CA syndrome in a rat model (Hayashida et al., 2012).
H2 also mitigated damage during the transplantation of various organs by H2 gas (Buchholz et al., 2008), or H2-water (Cardinal et al., 2010) and H2-preservation solution (Noda et al., 2013).
Ono et al. intravenously administered H2-saline with Edaravone, a clinically approved radical scavenger, in 8 patients with acute brain stem infarction and compared magnetic resonance imaging (MRI) indices of 26 patients who received Edaravone alone. The relative diffusion-weighted images (rDWIs), regional apparent diffusion coefficients (rADCs), and pseudo-normalization time of rDWI and rADC were all improved with the combined infusion of H2 with Edaravone (Ono et al., 2011).
3. Protective effects against neurodegeneration
Chronic oxidative stress is widely accepted as one of the causes of neurodegeneration, including dementia and Parkinson disease (PD) (Andersen, 2004, Federico et al., 2012). Experimental oxidative stress in the brain can be induced by chronic physical restraint stress and can impair learning and memory (Liu et al., 1996, Abrous et al., 2005). Additionally, neural proliferation in the dentate gyrus of the hippocampus is suppressed by the restraint stress (Abrous et al., 2005). Drinking H2-water suppressed the increase in this oxidative stress and prevented this cognitive impairment (Nagata et al., 2009). Moreover, H2-water restored neural proliferation in the dentate gyrus of the hippocampus in this model animal (Nagata et al., 2009). Since antidepressants increase adult neurogenesis (Becker and Wojtowicz, 2007, Sahay and Hen, 2007), H2-water may be applicable for improving depression and some mental disorders.
In PD, mitochondrial dysfunction and the associated oxidative stress are major causes of dopaminergic cell loss in the substantia nigra (Yoritaka et al., 1996, Schapira, 2008). H2 in drinking water was given before or after stereotactic surgery for 6-hydroxydopamine-induced nigrostrital degeneration in a rat model of PD. H2-water prevented both the development and progression of nigrostriatal degeneration in rats (Fu et al., 2009). Moreover, drinking H2-water also suppressed dopaminergic neuronal loss in another PD mice model induced by MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) (Fujita et al., 2009).
In a placebo-controlled, randomized, double-blind, parallel-group clinical pilot study, the efficacy of H2-water in patients with levodopa-medicated PD was assessed (Yoritaka et al., 2013). Participants drank 1 L per day of H2-water or pseudo water for 48 weeks. Total Unified Parkinson’s Disease Rating Scale (UPDRS) scores in the H2-water group (n = 9) improved, whereas UPDRS scores in the placebo group (n = 8) worsened. Despite the low number of patients and the short duration of the trial, the difference was significant (P < .05).
4. Preventive effects against metabolic syndrome
Drinking H2-water stimulates energy metabolism (Kamimura et al., 2011). H2-water significantly alleviated fatty liver in db/db mice, which is a type 2 diabetes model mice with obesity, as well as high fat-diet-induced fatty liver in wild-type mice. Long-term H2-water drinking significantly decreased fat and body weights, despite no increase in the consumption of diet and water, in db/db mice. Moreover, drinking H2-water decreased levels of plasma glucose, insulin, and triglyceride by stimulating energy metabolism (Kamimura et al., 2011).
Beneficial roles of H2-water in the prevention of potential metabolic syndrome were reported by 3 independent clinical studies as follows.
Kajiyama et al. performed a randomized, double-blinded, placebo-controlled, crossover study in 30 patients with diabetes mellitus type II and 6 patients with impaired glucose tolerance. The patients consumed either 900 mL H2-water or placebo water for 8 weeks, with a 12-week washout period. Statistical significance was observed in the improvement of electronegative charge-modified low-density lipoprotein (LDL)-cholesterol, small dense LDL, and urinary 8-isoprostanes. In four of six patients with impaired glucose tolerance, H2 improved their indexes of the oral glucose tolerance test to a normal level (Kajiyama et al., 2008).
Nakao et al. performed an open-label trial in 20 subjects with potential metabolic syndrome (Nakao et al., 2010b). The participants drank H2-water (1.5–2.0 L/day) for 8 weeks and showed an increase in urinary SOD; a decrease in urinary thiobarbituric acid reactive substances (TBARS), a marker of lipid peroxidation; an increase in high-density-lipoprotein (HDL)-cholesterol; and a decrease in total cholesterol.
Song et al. characterized the effects of H2-water (0.9–1.0 L/day) on the content, composition, and biological activities of serum lipoproteins in 20 patients with potential metabolic syndrome. Serum analysis showed that drinking H2-water for 10 weeks resulted in decreased serum total-cholesterol (TC) and LDL-cholesterol levels. In addition, H2-water significantly improved i) protection against LDL oxidation, ii) inhibition of tumor necrosis factor-α (TNF-α)-induced monocyte adhesion to endothelial cells, iii) stimulation of cholesterol efflux from macrophage foam cells, and iv) protection of endothelial cells from TNF-α-induced apoptosis (Song et al., 2013).
Taken together, these results strongly suggest the benefits of drinking H2-water in patients with metabolic syndrome.
5. Suppressive effects on inflammation
H2 reduced inflammation in experimental model animals induced by concanavalin A (Kajiya et al., 2009), dextran sodium sulfate (Kajiya et al., 2009), lipopolysaccharide (LPS) (Xu et al., 2012, Chen et al., 2013), Zymosan, an inducer of generalized inflammation (Xie et al., 2010b), and polymicrobial sepsis (G.M. Li et al., 2013). H2 gas, H2-saline and H2-water decreased the levels of pro-inflammatory cytokines to suppress inflammation.
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by the destruction of bone and cartilage. Ishibashi et al. administered H2-water containing 4–5 mg/L H2 (high H2-water) (0.5 L/day) for 4 weeks to 20 patients with RA. Urinary 8-hydroxy-deoxyguanine (8-OHdG) was significantly reduced by 14.3% (P < 0.01) on average. Disease activity (DAS28, using C-reactive protein levels also decreased (P < 0.01) during the same period. After the washout period, both the urinary 8-OHdG and the mean DAS28 had decreased compared to the end of the drinking period. During the second drinking period, the mean DAS28 was reduced (P < 0.01). All the 5 patients with early RA (duration < 12 months) who did not show antibodies against cyclic citrullinated peptides (ACPAs) achieved remission, and 4 of them became symptom-free at the end of the study. Thus, the symptoms of RA were significantly improved with H2-water (Ishibashi et al., 2012, Ishibashi, 2013).
6. Mitigation of side effects in treatments of cancers
Inhalation of H2 gas and drinking H2-water improved mortality and body-weight loss caused by treatment with an anti-cancer drug, cisplatin, and alleviated nephrotoxicity in mice. Despite its protective effects against cisplatin-induced toxicity, H2 did not affect the anti-tumor activity of cisplatin against cancer cell lines in vitro and tumor-bearing mice in vivo (Nakashima-Kamimura et al., 2009).
Kang et al. performed a randomized placebo-controlled clinical study of H2-water (0.55–0.65 mM, 1.5–2.0 L/day) for 6 weeks in 49 patients receiving radiation therapy for malignant liver tumors. H2 suppressed the elevation of total hydroperoxide levels, maintained serum antioxidant capacity, and improved the quality of life (QOL) scores. In particular, H2-water efficiently prevented loss of appetite. There was no difference in tumor response to radiotherapy between the two groups (Kang et al., 2011).
7. Effects on dermatomyositis and mitochondrial diseases
Ito et al. performed an open-label trial of H2-water (1.0 L/day) for 12 weeks in 14 patients with muscular diseases, including muscular dystrophies, polymyositis/dermatomyositis, and mitochondrial myopathies. In the open-label trial, significant improvements were observed in the lactate:pyruvate ratio, fasting blood glucose, serum matrix metalloproteinase-3 (MMP3), and triglycerides. In particular, the lactate:pyruvate ratio, which is a sensitive biomarker of a compromised mitochondrial electron transport system, was decreased by 28% in mitochondrial myopathies. In addition, MMP3, which represents the activity of inflammation, was decreased by 27% in dermatomyositis (Ito et al., 2011).
Then, they performed a randomized, double-blind, placebo-controlled, crossover trial of H2-water or placebo-dehydrogenized water (0.5 L/day) for 8 weeks in 22 patients with dermatomyositis and mitochondrial myopathies. In the double-blind trial, a statistically significant improvement was observed only in serum lactate in mitochondrial myopathies by H2-water consumption, but the lactate:pyruvate ratio in mitochondrial myopathies and MMP3 in dermatomyositis also tended to be decreased by H2-water consumption (Ito et al., 2011).
8. Mitigating effects of hemodialysis
Nakayama et al. performed an open-label placebo-controlled crossover trial of 12 sessions of hemodialysis in eight patients (Nakayama et al., 2009, Nakayama et al., 2010) and an open-label trial of 78 sessions of hemodialysis in 21 patients (Nakayama et al., 2010). In both studies, continuous sessions of hemodialysis with H2-dialysis solution decreased systolic blood pressure before and after dialysis. In the short-term study, plasma methylguanidine was significantly decreased. In the long-term study, plasma monocyte chemoattractant protein 1 and myeloperoxidase were significantly decreased.
9. Effects on acute erythematous skin diseases
Ono et al. treated 4 patients with acute erythematous skin diseases with fever and/or pain by intravenous administration of 0.5 L of H2-fluid for 30 min for more than 3 days. Erythema of these 4 patients and associated symptoms improved significantly after H2 treatment. In conclusion, an improvement in acute erythemtous skin diseases followed the administration of H2-fluid without compromising safety (Ono et al., 2012b).
10. Effects on exercise
Aoki et al. recruited ten young male soccer players and subjected them to exercise tests and blood sampling. Each subject was examined twice in a crossover double-blind manner; they were given H2-water or placebo water for one-week intervals. Subjects were requested to use a cycle ergometer at 75 % maximal oxygen uptake (VO2) for 30 min, followed by measurement of peak torque and muscle activity throughout 100 repetitions of maximal isokinetic knee extension. Acute exercise resulted in an increase in blood lactate levels in the subjects given placebo water, whereas oral intake of H2-water prevented the elevation of blood lactate during heavy exercise. Peak torque in the placebo water-group significantly decreased during maximal isokinetic knee extension, indicating muscle fatigue (Aoki et al., 2012).
Comparison with the other medical gasses
Several medical gasses are expected to provide more effective therapeutic interventions and preventive medicine. Hydrogen sulfide (H2S), carbon monoxide (CO), and  NO are extremely toxic molecules; however, they play important roles as signaling molecules (Kimura, 2010, Motterlini and Otterbein, 2010). CO is poisonous even in low concentrations due to its ability to interfere with oxygen delivery, whereas endogenous CO is important in the physiological functioning of organs and possesses anti-inflammatory properties (Motterlini & Otterbein, 2010). In contrast, H2 is advantageous to have no cytotoxicity.
NO are extremely toxic molecules; however, they play important roles as signaling molecules (Kimura, 2010, Motterlini and Otterbein, 2010). CO is poisonous even in low concentrations due to its ability to interfere with oxygen delivery, whereas endogenous CO is important in the physiological functioning of organs and possesses anti-inflammatory properties (Motterlini & Otterbein, 2010). In contrast, H2 is advantageous to have no cytotoxicity.
 NO are extremely toxic molecules; however, they play important roles as signaling molecules (Kimura, 2010, Motterlini and Otterbein, 2010). CO is poisonous even in low concentrations due to its ability to interfere with oxygen delivery, whereas endogenous CO is important in the physiological functioning of organs and possesses anti-inflammatory properties (Motterlini & Otterbein, 2010). In contrast, H2 is advantageous to have no cytotoxicity.
NO are extremely toxic molecules; however, they play important roles as signaling molecules (Kimura, 2010, Motterlini and Otterbein, 2010). CO is poisonous even in low concentrations due to its ability to interfere with oxygen delivery, whereas endogenous CO is important in the physiological functioning of organs and possesses anti-inflammatory properties (Motterlini & Otterbein, 2010). In contrast, H2 is advantageous to have no cytotoxicity.In the past decade, there has been marked, rapid growth in the knowledge of gaseous molecules. Gas inhalation as disease therapy has received recent interest (Szabó, 2007, Kajimura et al., 2010). It has recently been revealed that heme-based proteins play central roles in their generation- and reception-mechanisms as the primary target of these gas molecules. An integrated approach to the interaction of these gasses revealed the physiological significance of H2S, CO, and  NO on mitochondrial cytochrome c oxidase, a key target and central mediator of mitochondrial respiration (Kajimura et al., 2010). As far as briefly examined (Ohsawa et al., 2007), H2 did not reduce the oxidized heme of cytochrome c. Thus, the primary target of H2 seems to differ from that of the other medical gasses.
NO on mitochondrial cytochrome c oxidase, a key target and central mediator of mitochondrial respiration (Kajimura et al., 2010). As far as briefly examined (Ohsawa et al., 2007), H2 did not reduce the oxidized heme of cytochrome c. Thus, the primary target of H2 seems to differ from that of the other medical gasses.
 NO on mitochondrial cytochrome c oxidase, a key target and central mediator of mitochondrial respiration (Kajimura et al., 2010). As far as briefly examined (Ohsawa et al., 2007), H2 did not reduce the oxidized heme of cytochrome c. Thus, the primary target of H2 seems to differ from that of the other medical gasses.
NO on mitochondrial cytochrome c oxidase, a key target and central mediator of mitochondrial respiration (Kajimura et al., 2010). As far as briefly examined (Ohsawa et al., 2007), H2 did not reduce the oxidized heme of cytochrome c. Thus, the primary target of H2 seems to differ from that of the other medical gasses.Regarding the interaction between H2 and the other toxic medical gasses, combined therapy with H2 and CO demonstrated enhanced therapeutic efficacy via both anti-oxidant and anti-inflammatory mechanisms, and may be a clinically feasible approach for preventing ischemia/reperfusion injury in the myocardium (Nakao et al., 2010a).
Breathing NO plus H2 during ischemia/reperfusion reduced the infarct size and maintained cardiac function, and reduced the generation of myocardial nitrotyrosine associated with  NO inhalation. Administration of
NO inhalation. Administration of  NO plus H2 gasses for inhalation may be useful for planned coronary interventions or for the treatment of ischemia/reperfusion injury (Shinbo et al., 2013).
NO plus H2 gasses for inhalation may be useful for planned coronary interventions or for the treatment of ischemia/reperfusion injury (Shinbo et al., 2013).
 NO inhalation. Administration of
NO inhalation. Administration of  NO plus H2 gasses for inhalation may be useful for planned coronary interventions or for the treatment of ischemia/reperfusion injury (Shinbo et al., 2013).
NO plus H2 gasses for inhalation may be useful for planned coronary interventions or for the treatment of ischemia/reperfusion injury (Shinbo et al., 2013).The production of  NO, H2S or CO is catalyzed by
NO, H2S or CO is catalyzed by  NO synthases, cystathionine γ-lyase and cystathionine β-synthase or HO-1, respectively (Kashfi & Olson, 2013). In contrast, mammalian cells have no enzyme for producing intracellular H2.
NO synthases, cystathionine γ-lyase and cystathionine β-synthase or HO-1, respectively (Kashfi & Olson, 2013). In contrast, mammalian cells have no enzyme for producing intracellular H2.
 NO, H2S or CO is catalyzed by
NO, H2S or CO is catalyzed by  NO synthases, cystathionine γ-lyase and cystathionine β-synthase or HO-1, respectively (Kashfi & Olson, 2013). In contrast, mammalian cells have no enzyme for producing intracellular H2.
NO synthases, cystathionine γ-lyase and cystathionine β-synthase or HO-1, respectively (Kashfi & Olson, 2013). In contrast, mammalian cells have no enzyme for producing intracellular H2.Concluding remarks
This article reviewed the progress of hydrogen medicine from its initiation toward clinical applications. H2 is easily applicable because it has no adverse effects and great efficacy on nearly all pathogenic states involved in oxidative stress and inflammation. Indeed, the clinical effects of H2 were positive in patients with more than 10 various diseases. Since most pharmacological drugs specifically act on their targets, H2 seems to differ from conventional drugs because of its extensive and various effects. H2 has great potential for preventive and therapeutic applications in many diseases owing to its great efficacy and its novel concept.



