Author: Guohua Song, Min Li, Hui Sang,Liying Zhang, Xiuhong Li,Shutong Yao, Yang Yu, Chuanlong Zong, Yazhuo Xue, Shucun Qin July 2013
Abstract
We have found that hydrogen (dihydrogen; H2) has beneficial lipid-lowering effects in high-fat diet-fed Syrian golden hamsters. The objective of this study was to characterize the effects of H2-rich water (0.9–1.0 l/day) on the content, composition, and biological activities of serum lipoproteins on 20 patients with potential metabolic syndrome. Serum analysis showed that consumption of H2-rich water for 10 weeks resulted in decreased serum total-cholesterol (TC) and LDL-cholesterol (LDL-C) levels. Western blot analysis revealed a marked decrease of apolipoprotein (apo)B100 and apoE in serum. In addition, we found H2 significantly improved HDL functionality assessed in four independent ways, namely, i) protection against LDL oxidation, ii) inhibition of tumor necrosis factor (TNF)-α-induced monocyte adhesion to endothelial cells, iii) stimulation of cholesterol efflux from macrophage foam cells, and iv) protection of endothelial cells from TNF-α-induced apoptosis. Further, we found consumption of H2-rich water resulted in an increase in antioxidant enzyme superoxide dismutase and a decrease in thiobarbituric acid-reactive substances in whole serum and LDL. In conclusion, supplementation with H2-rich water seems to decrease serum LDL-C and apoB levels, improve dyslipidemia-injured HDL functions, and reduce oxidative stress, and it may have a beneficial role in prevention of potential metabolic syndrome.
RESULTS
Subject characteristics
The baseline demographics of subjects are presented in Table 1. Subjects enrolled in the study included those who had TC > 5.18 mmol/l (n = 17), LDL-C >2.59 mmol/l (n = 18), BMI 25–34.9 (n = 17), and/or smokers (n = 10). All subjects showed mean normal clinical levels of baseline biometric parameters, clinical chemistry, and hematology. All smokers were occasional smokers.
Effect of H2 on serum lipoprotein profiles and serum levels of antioxidative and inflammatory biomarkers
The serum lipid levels of each individual are presented in Table 2, and the distribution of lipid levels is shown in Fig. 1. We can see that serum TC and LDL-C levels were significantly decreased after 10 weeks of H2 treatment in all of the 20 patients with potential metabolic syndrome. Among 20 patients, 18 had decreased and 1 (patient 14) had increased TC and LDL-C levels, and 1 (patient 8) was not altered after H2 consumption.
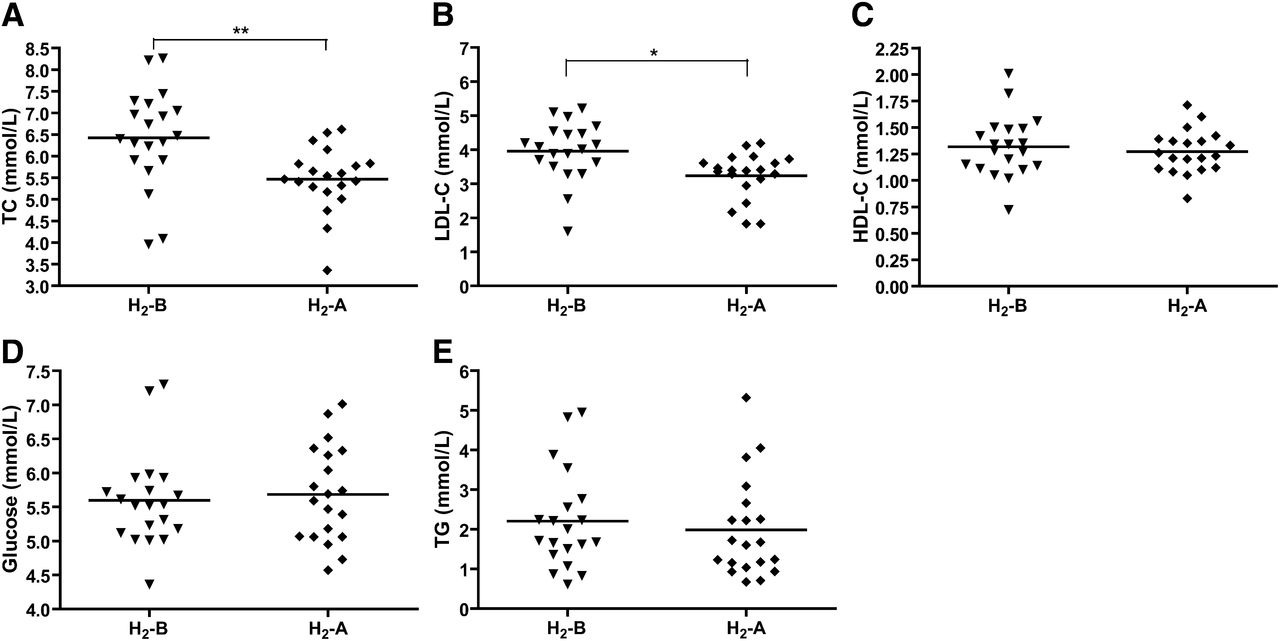
Fig. 1 Effect of H2 on the levels of serum lipids and glucose. (A) Serum TC, (B) LDL-C, (C) HDL-C, (D) glucose, and (E) TG in patients with potential metabolic syndrome before and after 10 weeks of H2 consumption were determined by enzymatic method. Data (means ± SD, n = 20) were expressed as mmol per liter. H2-B, before drinking H2-rich water; H2-A, after drinking H2-rich water. *P < 0.05, **P < 0.01.
TABLE 2: https://www.jlr.org/action/showFullTableHTML
Effect of H2 on levels of serum lipids and glucose
* P < 0.05 compared with TC levels before drinking H2-rich water;
** P < 0.01 compared with TC levels before drinking H2-rich water;
# P < 0.05 compared with LDL-C levels before drinking H2-rich water. M, male; F, female.
In addition, among the 20 patients, 10 were smokers and 10 were nonsmokers. As shown in Table 2, H2 treatment decreased serum TC and LDL-C levels not only in smokers but also in nonsmokers. And it seems that the lipid-lowering effects of H2 on smokers were better than those on nonsmokers, although there is no significant difference. Serum levels of HDL-C and glucose were not altered by consumption of H2 (Table 2 and Fig. 1C, D). Interestingly, serum TG levels were decreased by H2 treatment in 10 smokers, although there is no significant difference (Table 2), which caused a slight decrease in TG levels in all of the 20 patients (Table 2 and Fig. 1E).
Moreover, serum lipoprotein profile by FPLC further confirmed the decrease in serum LDL-C levels in H2-treated subjects and revealed that serum VLDL-C and HDL-C remained unchanged after intake of H2 water (Fig. 2).

Fig. 2 FPLC cholesterol profiles using pooled serum samples of n = 5 patients per group showing cholesterol content (mg/dl) of serum lipoprotein fractions.
Changes in biomarkers of oxidative and inflammatory status after 10 weeks of consumption of H2 water are shown in Fig. 3. Serum levels of MDA, one of the most frequently used indicators of lipid peroxidation, were decreased significantly (Fig. 3A), and the activity of SOD, which acts as an antioxidant and protects cellular components from being oxidized by reactive oxygen species, was increased after H2 water consumption (Fig. 3B). However, the activity of PON1, an antioxidant enzyme associated with HDL, was not altered in either serum or HDL3 fractions by H2 water (Fig. 3C, D). In addition, we detected serum concentrations of several biologically active oxidized lipids, including 12-HETE, 13-HODE, PG, and 8-iso-PGF2α. The data in Fig. 3E, F shows that 13-HODE and 8-iso-PGF2α levels were improved following the H2 treatment; however, 12-HETE and PG levels were not altered by H2. There was no significant effect of intake of H2 water on serum levels of inflammatory biomarkers, including TNF-α and IL-6 (Fig. 3G, H).
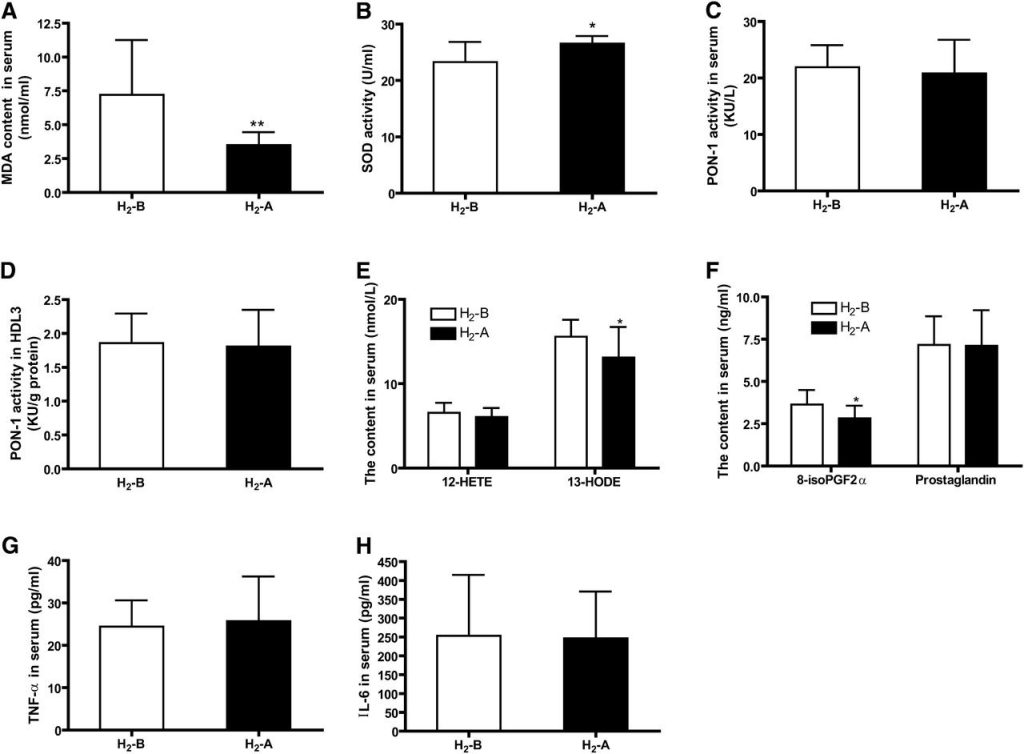
Fig. 3 Effect of H2 on serum levels of antioxidative and inflammatory biomarkers. (A) Serum concentrations of MDA. (B) SOD activity in serum. (C and D) PON1 activity in serum and HDL3 fraction. (E) Serum concentrations of 12-HETE and 13-HODE. (F) Serum concentrations of 8-iso-PGF2α and PG. (G) Serum concentrations of TNF-α. (H) Serum concentrations of IL-6. n = 20, *P < 0.05, **P < 0.01.
H2 treatment decreases apoB and apoE protein levels in serum
Serum LDL and HDL are particles composed of a variety of lipids and protein components; thus, it is necessary to clarify which content of the lipoprotein could be affected by H2 treatment. Consistent with the difference observed for LDL-C, apoB100, the major protein on LDL, was significantly decreased by consumption of H2 water in patients with potential metabolic syndrome (Fig. 4). The apoE protein, which is mainly present in VLDL and LDL particles, was also lowered with the treatment of H2 (Fig. 4). ApoAI, the major protein on HDL, was not altered after intake of H2 water (Fig. 4), which is consistent with the changes observed for HDL-C. These data suggested that H2 could decrease the expression of the major protein constituents of LDL and VLDL.
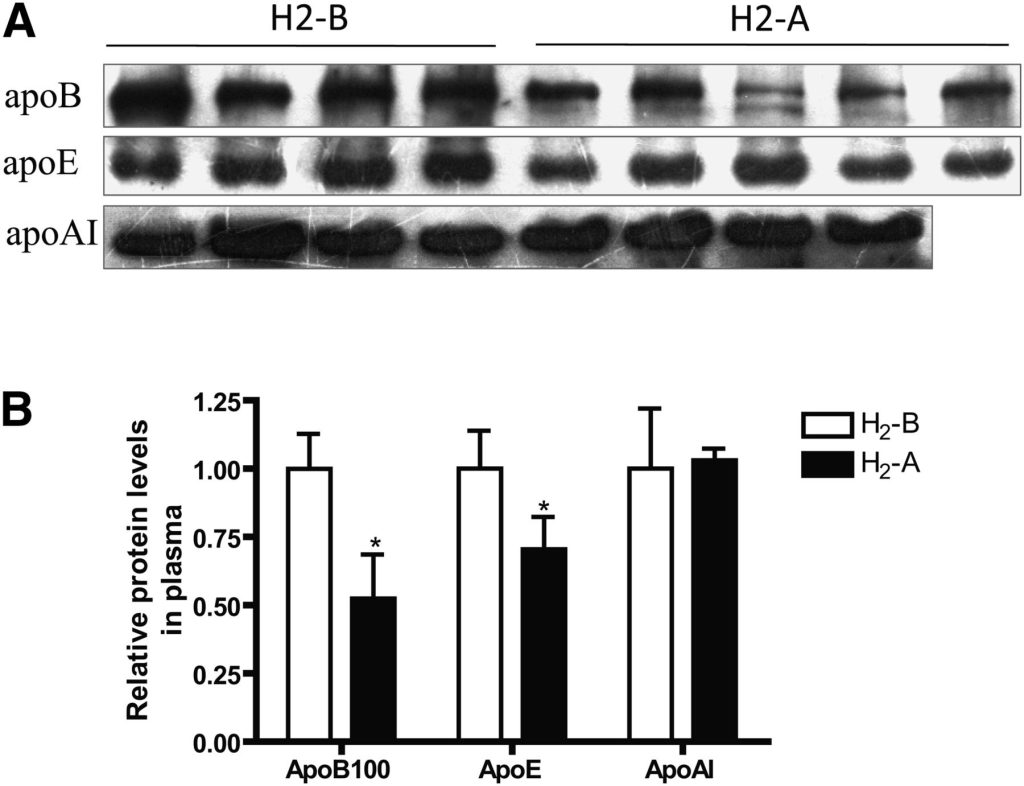
Fig. 4 Effect of H2 on serum levels of apoB, apoE, and apoAI proteins. (A) Effect of H2 on serum apoB, apoE, and apoAI protein levels by western blots. (B) Densitometric quantitation of western blot data (n = 4–5) by Quantity One software. *P < 0.05.
H2 improves the oxidation and the functional properties of the HDL particle
HDL is known to undergo dramatic modification in structure and composition under the concerted actions of inflammation and oxidative stress (14, 15). Recent evidence indicates that H2 acts as a therapeutic medical gas in a variety of disease models by exerting antioxidant and anti-inflammatory effects (3, 25, 26). Therefore, it is possible that administration of H2 might improve the functional quality of HDL particle. First, H2 water supplementation tended to decrease the MDA content in HDL3 (Fig. 5A), suggesting that H2 improves the oxidation of HDL particle.
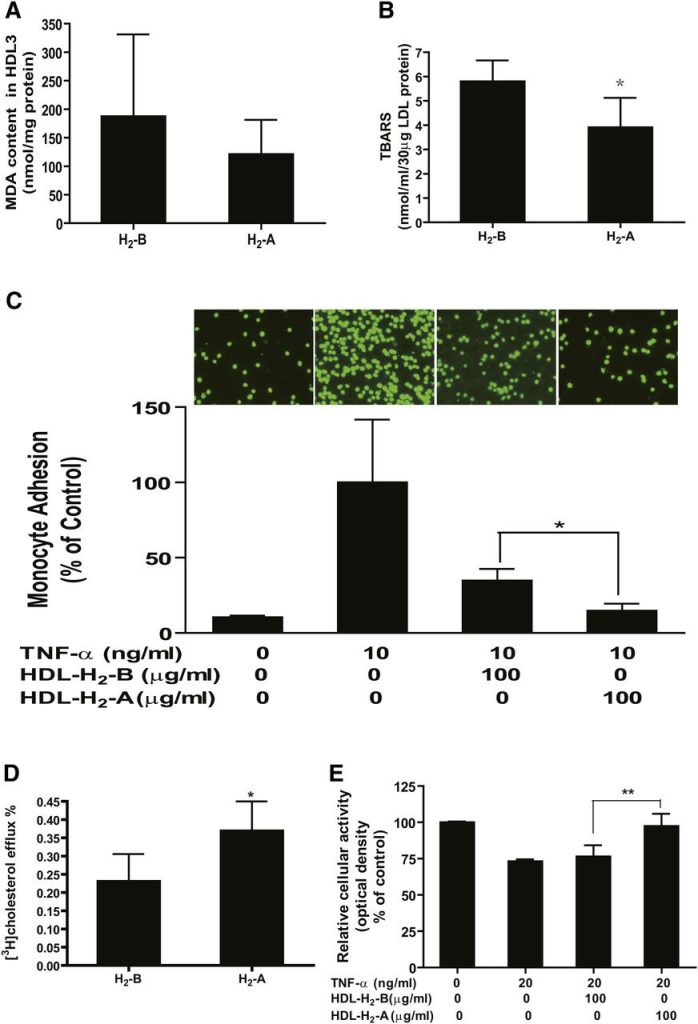
Fig. 5 H2 seems to improve the oxidation and functional properties of the HDL particle. Serum of every 5–6 patients was pooled, and HDL3 was isolated by ultracentrifugation from the serum. (A) MDA content in HDL3 particles. HDL function was determined as (B) protection of LDL against oxidation, (C) inhibition of TNF-α-induced THP-1 monocyte adhesion to endothelial cells, (D) stimulation of cholesterol efflux from macrophage foam cells, and (E) protection of endothelial cells from TNF-α-induced apoptosis. Assays were performed as detailed in Methods. n = 3–4 pooled serum samples. *P < 0.05, **P < 0.01.
Second, the biological effect of H2 on the antioxidative functionality of HDL was tested, namely, the protection of LDL particles from oxidation. As shown in Fig. 5B, H2 treatment significantly inhibited the formation of TBARS.
Third, the effect of H2 on the anti-inflammatory properties of HDL was tested, including protection of cytokine-induced monocyte adhesion to endothelial cells and stimulation of endothelial nitric oxide production, which has been suggested as an important endothelial-atheroprotective effect of HDL. As shown in Fig. 5C, after incubation of HUVECs for 6 h with TNF-α, adhesion of monocytes to HUVECs was significantly increased, and preincubation of HUVECs with HDL3 isolated from patients after 10 weeks of drinking H2 water (HDL-H2-A) markedly reduced TNF-α-induced adhesion of monocyte to HUVECs compared with those of HDL3 isolated at baseline (0 week) (HDL-H2-B). These data suggest that the anti-inflammatory function of HDL was improved by H2 water. We also determined the effects of HDL-H2-A compared with HDL-H2-B on endothelial nitric oxide production. Unfortunately, the nitric oxide production was not altered significantly after intake of H2 water (data not shown), which might be attributable to the sensitivity of Griess method.
Fourth, the ability of the isolated HDL particles to elicit efflux from cholesterol-loaded macrophages was tested. As shown in Fig. 5D, HDL particles isolated from the serum after H2 treatment exhibited dramatically higher efflux properties compared with the HDL particles isolated from the serum before H2 treatment, indicating that the cholesterol efflux ability mediated by HDL particles was increased by H2.
Finally, the biological effect of H2 on the antiapoptotic functionality of HDL was determined. As shown in Fig. 5E, H2 treatment significantly inhibited TNF-α induced endothelial cell apoptosis. These data indicate that dyslipidemia-injured HDL functions, including the ability to protect against LDL oxidation, the ability to inhibit cytokine-induced monocyte adhesion to endothelial cells, the ability to stimulate cholesterol efflux from macrophage foam cells, and the ability to protect endothelial cell apoptosis, were markedly improved by administration of H2 water in patients with potential metabolic syndrome.
H2 reduces the oxidation of LDL and LDL-mediated inflammation
The oxidation of LDL plays an important role in atherogenesis and may influence lipid metabolism. In this study, we determined the effects of H2 water on the oxidation of LDL and LDL-mediated inflammation. As shown in Fig. 6A, the MDA content of the isolated LDL was reduced by H2 treatment, suggesting that H2 reduces the oxidation of LDL. To determine LDL-mediated inflammation, 100 μg protein/ml of the isolated LDL was added to the cultured RAW264.7 macrophages and bone marrow-derived macrophages for 24 h. As shown in Fig. 6B, C, the secretion of TNF-α and IL-6 by macrophages was reduced by H2 treatment. Furthermore, we tested the effects of H2 on LDL-induced monocyte adhesion to endothelial cells. As shown in Fig. 6D, consumption of H2 water significantly decreased LDL-induced monocyte adhesion to endothelial cells. These data reveal that H2 reduces LDL-mediated inflammatory properties in culture.
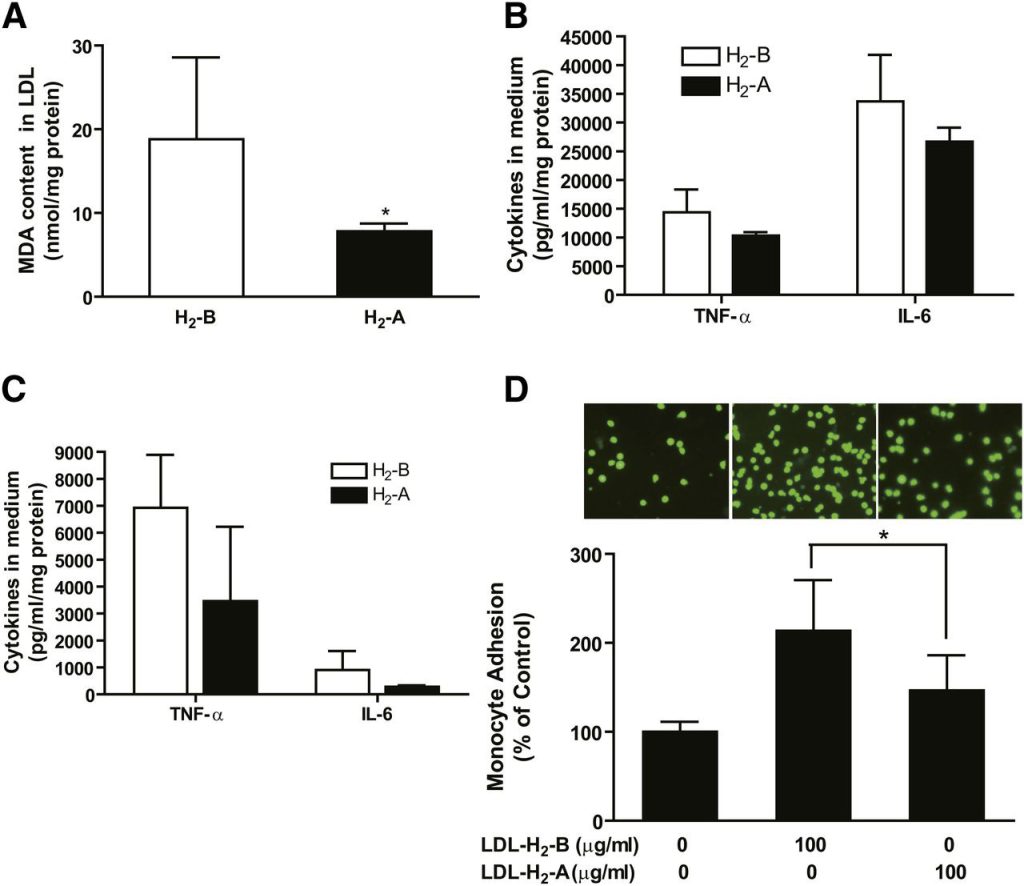
Fig. 6 H2 seems to reduce oxidation of LDL in patients and LDL-mediated inflammation in macrophages. Serum of every 5–6 patients was pooled, and LDL was isolated by ultracentrifugation from the serum. (A) MDA content of the isolated LDL. (B) One hundred micrograms of protein per milliliter of isolated LDL was added to cultured RAW264.7 macrophages. Supernatants were harvested at 24 h for measurement of TNF-α and IL-6 by ELISA assay. (C) One hundred micrograms of protein per milliliter of isolated LDL was added to the cultured bone marrow-derived macrophages, and cytokines in medium were measured after 24 h incubation. (D) HUVECs were stimulated with LDL (100 μg protein/ml) for 6 h and THP-1 cells, labeled with a fluorescent dye BCECF-AM, were added to HUVECs. After adhering, THP-1 cells bound to HUVECs were counted under fluorescent microscope as described in Methods. Results are expressed as means ± SD (n = 3–4 pooled serum samples). *P < 0.05.
DISCUSSION
In a previous study, we found that H2 has beneficial lipid-lowering effects in high-fat diet-fed Syrian golden hamsters (6). However, it remains unknown whether H2 has effects on lipid and lipoprotein metabolism in humans. The key finding of our present study is that the novel antioxidant chemical element H2 seems to alleviate lipid metabolism disorder, including hyperlipidemia and defective HDL function, in patients with potential metabolic syndrome. Our results were not consistent with that of human studies in type 2 diabetes mellitus, as they showed significant decreases in modified LDL-C levels and no effect on total and LDL-C (3). The inconsistency might be attributable to the difference in pathological conditions, the dosage of administration, or the intervention period. Despite this possibility, the discrepancy might be explained by the fact that modified LDL, like oxidized LDL, largely existed in the hyperlipidemia model, which was used in our study.
Subanalysis was conducted on 20 subjects, and we found the serum TC levels of 17 subjects with hyperlipidemia (TC > 5.18 mmol/l) was decreased by intake of H2 water for 10 weeks, and the serum TC levels of the remaining 3 subjects without hyperlipidemia (TC < 5.18 mmol/l) was not significantly altered by H2 treatment, although the TC level of 1 subject was slightly increased after treatment (Table 2, patient 14). The data gave us a clue that H2 might exert a lipid-lowering effect on the patients with hyperlipidemia but have little effect on the population without hyperlipidemia.
It is well known that high cholesterol level is one of the important risk factors for atherosclerosis. Therefore, the elucidation of the mechanism by which H2 decreases serum cholesterol will provide solid evidence for the application of H2 in cardiovascular disease therapy. Serum LDL and VLDL are particles composed of a variety of lipids and protein components; thus, it is necessary to clarify which content of the lipoprotein could be affected by H2 treatment. First, LDL is a contributing factor to the development of atherosclerosis. ApoB100 is the major protein present in LDL particles, and like LDL-C, the serum apoB level has been positively correlated with risk for atherosclerotic disease. In the present study, we found H2 treatment not only decreased serum LDL-C levels, but also remarkably lowered apoB100 and apoE protein levels in serum. The data gave us a clue that H2 might regulate the metabolism of LDL-C by decreasing the synthesis of apoB or increasing the catabolism of LDL-C. Second, like LDL-C, VLDL-C is considered a type of ‘‘bad’’ cholesterol because elevated levels are associated with an increased risk of coronary artery disease (27). We found that apoB and apoE, the major proteins in VLDL, were lowered by H2, although cholesterol analysis by FPLC revealed no changes of VLDL-C after H2 treatment. The inconsistencies in protein and cholesterol levels might be explained by the suspicion that the functional target of H2 might be apolipoprotein expression, not cholesterol. Taken together, the data indicate that molecular H2 dissolved in water might have a beneficial regulating effect on lipid abnormality in patients with metabolic syndrome, especially in patients with hyperlipidemia. This effect is partially related to its regulation of lipid and protein contents of LDL and VLDL. Further experiments are needed to identify the mechanisms by which H2 regulates the lipid and protein contents of lipoprotein particles and improves the serum lipoprotein profile. Previous studies have demonstrated that administration of H2-rich saline improves insulin sensitivity (3), which, in our view, could partly contribute to the improved lipid metabolism in our study. Furthermore, liver cells sense the reduced levels of hepatic intracellular cholesterol and seek to compensate by synthesizing LDL receptors to draw cholesterol out of the circulation (28). Future studies on hepatic HMG-CoA reductase and LDL receptors may elucidate the mechanism by which H2 acts on lipoprotein regulation.
HDL is known to protect against the development of atherosclerosis and is widely documented as a “negative risk factor” for coronary heart diseases (29). The antiatherogenic activity of HDL is principally attributable to a variety of antioxidative, anti-inflammatory, and antiapoptotic properties and the reverse transport of cholesterol (30). In the present study, we that found H2 treatment seems to improve the functionality of HDL3 without altering HDL-C serum levels. It is known that the beneficial effects of H2 on different disease models are mostly dependent on its antioxidative, anti-inflammatory, and antiapoptotic properties (16). Therefore, it is possible that the protective effect of H2 on HDL function in our study is attributable, at least in part, to the antioxidative and anti-inflammatory properties of H2. A number of therapeutic strategies are being developed to target HDL-C in an attempt to inhibit the progression or induce regression of atherosclerosis and reduce cardiovascular events. Therefore, the protective effect of H2 on HDL function in the our study might provide a further evidence for H2 application in vascular disease therapy. We did not observe any unwanted side effects of H2, including headache, diarrhea, and vomiting (data not shown), suggesting fewer toxic and adverse effects of H2.
The oxidation of LDL plays an important role in atherogenesis and may influence lipid metabolism. Our data reveals that H2 seems to reduce the oxidation of LDL and LDL-mediated inflammation, and they further support our prediction that H2 might improve lipid metabolism in patients with hyperlipidemia by inhibiting LDL-mediated inflammation. Previous studies have demonstrated that administration of H2 reduces atherogenesis in apoE−/− mice (5); in our view, this could partly contribute to the observed protective effects of H2 on the oxidation of LDL and LDL-mediated inflammation in our study.
Indeed, our data give us a clue that H2 might have the potential to be used as a novel lipid-regulating agent with the advantage of no toxicity compared with other commonly used lipid-regulating drugs that have side-effects on the liver and kidney. Further understanding of the mechanisms underlying the signaling pathways involved in the ability of H2 to influence lipid and cellular metabolism is required to fully exploit intake of H2 gas as a therapeutic strategy.
There are several limitations to the present study. First, the number of subjects recruited is small and it is hard to draw a solid conclusion, particularly having smokers among the subjects. It should, however, be emphasized that we analyzed the data in smokers and nonsmokers separately, and the response to H2 in smokers seems to be stronger than that in nonsmokers, but the difference did not reach statistical significance. It would be useful to enlarge the sample size and examine the reaction to H2 in smokers due to the antioxidative property of H2. Another limitation of the study was that it was not a double-blind, randomized, controlled trial comparing H2-rich water to placebo and comparing experimental endpoints from H2-treated metabolic syndrome subjects with those measured in untreated healthy individuals. It is possible that during the 10 weeks of the study, the subjects altered their lifestyles, which could have affected the parameters studied. Therefore, it is difficult for us to draw a solid conclusion, and it limits the conclusions to stating that H2 treatment appear to regulate lipid metabolism in patients.
In summary, our data show that in vivo administration of H2 water seems to decrease serum TC and LDL-C levels and improve HDL functions in patients with potential metabolic syndrome, suggesting that H2 may be used as a newer pharmacological agent to treat or control lipid metabolism disorder.



